Abstract
Introduction: Daratumumab (DARA) is a human CD38-targeted monoclonal antibody that is approved as monotherapy and in combination with bortezomib (a proteasome inhibitor; PI) or immunomodulatory drugs (IMiD; lenalidomide or pomalidomide) and dexamethasone (d) in pts with relapsed or refractory multiple myeloma (RRMM). Carfilzomib (K) is a PI that is approved for RRMM as a single agent and in combination with lenalidomide and d. We hypothesized that the addition of DARA to Kd would provide additional clinical benefit for relapsed multiple myeloma pts. Here, we present data from the DARA + Kd arm of a multi-arm, phase 1b study (NCT01998971) that evaluated DARA in combination with various backbone therapies.
Methods: Eligible pts had an Eastern Cooperative Oncology Group score of ≤2, had left ventricular ejection fraction (LVEF) ≥40%, and were treated with 1-3 prior lines of therapy that included bortezomib and an IMiD. Only K-naïve pts were enrolled. Pts received DARA weekly for Cycles 1-2, every 2 weeks for Cycles 3-6, and every 4 weeks thereafter. K was given on Cycle 1 Day 1 (C1D1) at 20 mg/m2 and escalated to 70 mg/m2 on C1D8. K was administered weekly on Days 1, 8, and 15 of each cycle; d 40 mg was given weekly. Ten pts received standard first DARA dose (16 mg/kg) on C1D1; the remaining pts received a split first dose of DARA at 8 mg/kg on C1D1 and 8 mg/kg on C1D2. The primary endpoint was safety and tolerability of DARA + Kd. Overall response rate (ORR) was a secondary endpoint and was examined in the response-evaluable population (received ≥1 post-baseline disease assessment after study treatment) who were treated for >2 cycles or discontinued treatment.
Results: A total of 85 pts were enrolled. The median (range) age was 66.0 (38-85) years. The median number of prior therapies received was 2 (1-4 [2 pts received 4 prior lines of therapy and were categorized as protocol deviations]); 99% received prior bortezomib, 94% received prior lenalidomide, 15% received prior pomalidomide, 73% received prior autologous stem cell transplant, 98% received a prior PI and IMiD, 60% were refractory to lenalidomide, and 29% were refractory to PI and IMiD. 20% of pts discontinued treatment due to disease progression (12%), adverse event (AE; 4%), pt withdrawal (4%), and physician decision (1% [the same pt was also categorized under pt withdrawal]).
At the clinical cutoff date (June 16, 2017), the most common (>10%) grade 3 or 4 treatment-emergent AEs (TEAEs) were thrombocytopenia (27%), lymphopenia (24%), anemia (20%), neutropenia (18%), and hypertension (12%). Serious TEAEs were observed in 32% of pts; 7% were related to DARA, 13% were related to K, and 12% were related to d. Treatment discontinuation due to TEAE was seen in 5% of pts. One death due to TEAE occurred (general health deterioration related to disease progression) and was unrelated to any of the study treatments. 60% of pts in the single-dose cohort (n = 10) and 40% of pts who received DARA in a split first dose (n = 75) experienced infusion-related reactions (IRRs), the majority of which were grade 1 or 2 in severity and occurred predominantly during the first infusion.
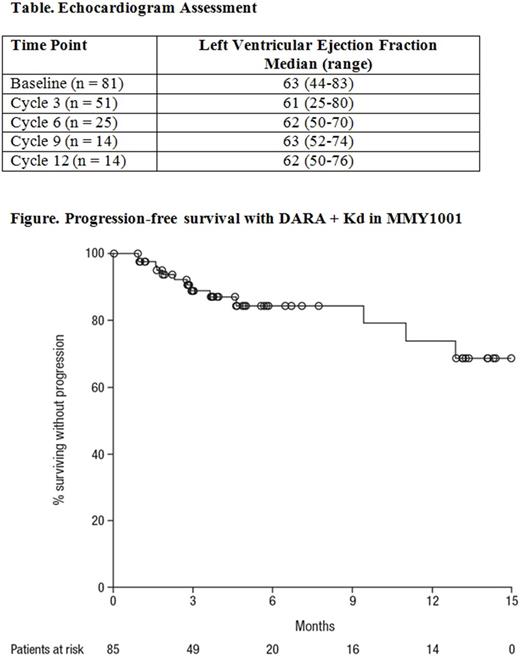
Median LVEF did not change over time from baseline (Table). At time of analysis, 3 pts with transient grade 3 cardiac AEs (cardiac failure, atrial fibrillation, and sinus tachycardia) recovered, and 1 pt with grade 3 congestive cardiomyopathy remained unresolved.
The overall median follow-up was 4.5 (range: 0.5-15.7) months. Among the response evaluable pts who received more than 2 cycles of treatment or discontinued early on, ORR was 84% (4% stringent complete response [CR]; 9% CR, 47% very good partial response, and 25% partial response). Median progression-free survival (PFS) was not reached (95% confidence interval [CI], 12.9 months-not estimable; Figure), and the 12-month PFS rate was 74% (95% CI, 54-86). Among pts that responded, the median duration of response was not reached (95% CI, 10 months-not estimable).
Updated safety and efficacy data will be presented at the meeting.
Conclusions: Addition of DARA to Kd resulted in a safety profile consistent with that of the individual therapies. Despite short follow-up, impressive responses were achieved in RRMM pts who were previously treated with standard of care agents (98% previously treated with PI + IMiD; 60% refractory to lenalidomide). A phase 3 study comparing DARA + Kd versus Kd in RRMM (CANDOR; NCT03158688) is ongoing.
San-Miguel: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Mateos: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Benboubker: Takeda, Celgene, Janssen, Amgen: Consultancy. Oriol: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Celgene: Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia. Arnulf: Janssen, Amgen, Celgene: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees. Chari: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Pharmacyclics: Research Funding; Array BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Bristol-Myers Squibb: Consultancy, Other: Research funding (to AC's institution); travel; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Acetylon Pharmaceuticals: Other: Research funding (to AC's institution); Biotest: Other: Research funding (to AC's institution); Onyx: Research Funding; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wu: Janssen: Employment. Wang: Janssen: Employment. Doshi: Janssen: Employment. Schecter: Janssen: Employment. Moreau: Millennium: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Honoraria; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria; Celgene, Janssen, Takeda, Novartis, Amgen, Roche: Membership on an entity's Board of Directors or advisory committees; Onyx Pharmaceutical: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal